



male brood pouch
copulation
full brood pouch
birth
fry and father
Identifying Patterns of Evolutionary Change
Outgroups: Polarizing Ancestral and Derived Traits
The Principle of Parsimony and the Construction of Phylogenetic Trees
Phylogenetic Inferences on Adaptation
Were Dinosaurs Dead-beat Dads?
The Evolution of Leks: Hotspot Hypothesis
Evolution of male parental care in sea horses and pipefish
Proximate and Ultimate Origins of Female Preferences
Stalk-eyed males originate as an ornament of quality used by females
Sensory Bias and Proximate Explanations for Phylogenetic Patterns
Throughout the readings we have focussed on a microevolutionary perspective regarding the evolution of behaviors. In our consideration of mechanisms of natural and sexual selection, we focussed on the behavioral traits being shaped by the success of individual organisms. The mechanisms of natural and sexual selection are referred to as microevolutionary processes. The power of the natural and sexual selection can lead to rapid evolution in a trait that is favored by adaptation. Adaptation is subject to constraints that limit the direction that evolution can take a species. Constraints on evolutionary change can arise at a number of levels. We have already focussed on constraints on adaptation that arise from the design of sensory structures, as well as the physical environment around the organism -- these are the constraints of proximate mechanisms.
A number of authors have advocated the idea that a species is constrained by its own phylogenetic history. During the evolution of a group, certain features were developed that served to limit the possible development of other features. For example, once birds developed wings and powered flight, this adaptation makes it necessary to limit the number of eggs that a female is carrying at any point in time to only a few eggs. Thus, most birds labor under the constraint that they cannot produce the eggs all at once like a lizard and they lay eggs in sequence one at a time. This constraint then makes it necessary to alter many other reproductive behaviors to make allowances for the fact that hatchlings might be of different age. Such phylogenetic constraints surely exist but they are difficult to identify. If birds evolved flight only once, then this gives evolutionary biologists on a single example to analyze, not a very large sample size. In addition, proof of the existence of such constraints relies on the comparative method. In most cases it is very difficult to experimentally manipulate the trait of interest. Such higher order processes that might limit or channel evolutionary change in certain directions are referred to as macroevolutionary processes.
We have already put the comparative method into practice when we ask questions concerning differences in male parental care among: sticklebacks, pipefish, and seahorses. We identified the changes in environment that may have been influential in promoting the evolution of male biased care. In the case of mating systems, any environmental factor that leads to a male versus a female biased operational sex ration will favor sexual selection on males versus females. What we lacked for a complete consideration of the comparative method was an idea of the history of evolutionary change in these groups. To understand the history of change, we have to develop a notion of phylogeny and cladistic relationships.
The fundamental concept underlying all phylogenetic analysis is the phylogenetic tree. The idea of a tree representing the evolutionary history of a group was so compelling to Darwin that he included a figure in his book the Origin of Species. Darwin was not one to include many pictures, as this is the only figure in the entire book. A phylogenetic tree traces genalogical relationships among species much like a family tree traces genalogical relationships among individuals. The formation of new lineages or species takes place by the Speciation Mechanisms that were described earlier.
Let us considered the topology and names for a few features on the tree to develop the working vocabulary of phylogenetic analysis. A branch point where it thought that two separate lineages arose is termed a node or the common ancestor of the members of the lineage. The group of species that are all descendants of the common ancestor are called a clade. Thus, the phylogenetic tree is also referred to as a cladogram in that it describes all ancestor-descendant relationships in a graphical form. The vertical axes for a cladogram reflects time.
Evolutionary biologists have many ways of assigning time to the vertical axis. The most intuitive component of time that we might imagine is real time as drawn from the fossil record. For example, the common ancestor of all homonid lineages is thought to be Australopithecus ramidus , an ape that appeared in the fossil record 4.6 million years ago. Australopithecus ramidus gave rise to the lineages of Australopithecines of which its most famous member, a skeleton called "Lucy", is thought to be a member of the species, Australopithecus afaraensis, that gave rise to all lineages of Homo as well as other branches of Australopithecus. In addition to fixing the vertical axis based on time, molecular biologists can calibrate the number of amino acid substitutions in a protein or the number of nucleotide base pairs in a gene with what is known as the molecular clock. If change in these molecules is constant over time and for all members of a clade, one could calibrate the molecular clock or rate of change in amino acid residues or base pairs using a single fossil that marked the divergence of two clades. Where the calibration is not available, information on genetic distance is used as a measure of "time".
The steps involved in any phylogenetic analysis are as follows:
The concept of an outgroup is crucial for interpreting the changes that might have occurred in a lineage. For example, an appropriate outgroup for hominid lineages would be the great apes. If you were to look at the behavior of the great apes (e.g., the genus Pan or chimpanzees) and found aspects of their behavior that is similar to modern representatives of the hominid lineages (just Homo sapiens), one might infer that such behaviors were also found in all extinct forms of the hominid lineages. The outgroup of chimpanzees presumably arose from the common ancestor in the remote past, and both humans and chimps share this common ancestor. Under Charles Darwin's paradigm of "descent with modification", one might assume that there was no modification in the transmission of behaviors. For example, consider the tool using abilities of humans. Jane Goodall has reported that chimpanzees use small twigs to get termites out of tree holes -- a clear indication of tool use. Is there evidence of tool use in fossils. Yes, good tools have been found associated with the fossils of Homo habilis and all later hominids. However, evidence for tool use in the Australopithecines is sketchy at best. But also notice that chimps use twigs, wooden tools, that are unlikely to fossilize, and even more unlikely for us to recognize them as tools even if we were to find them.
The hominid example serves to illustrate the limitations of any phylogenetic analysis. You cannot necessarily increase your sample size -- there is only one extant species of homo from which to draw information. However, there are more outgroups, that could be used to refine the information. For example, humans are thought to be most closely related to chimps. Gorillas are the species of great apes with which we share moderate relationships, and orangutans are furthest along. One could in prinicple date the divergence of gorillas, which do not use tools, from chimps and homo to perhaps date the origin of tool use in the family pongidae.
The phylogenetic argument implies that all Australopithecines used some kind of tools because tool use is shared by living members of clade (us) and our nearest outgroup -- the non-hominid chimpanzees. This is an argument based on inference, not direct observation of the ancestors of hominids. In many cases the condition of traits in the common ancestor are reconstructed from information provided by the outgroup. It is often assumed that the outgroup has more primitive traits than the clade of interest. Indeed, this feature of outgroup choice is often crucial to phylogenetic inference. This is because we are interested in two kinds of changes in a lineage. We are interested in clades that have:
A shared ancestral character is found in the outgroup, and in those members of the clade that have not experienced any modification of the trait from the state found in the common ancestor. This is because we infer that if the outgroup species shares the trait and it is found in some clades, the specis in these clades must have received the trait in an unmodified form from the common ancestor. An opposable thumb is found in all members of the pongidae, and it is generally thought that an opposable thumb is an important requirement for a tool using hominid (Note that other animals have evolved tool use, birds manipulate twigs with their beaks to obtain termites in much the same way as chimpanzees. Birds have found an alternative evolutionary pathway to tooluse.)
A shared derived character is ideally found in some subset of the clade and in nearly all members of that sub-clade. By inference we might hypothesize that those members of the clade with the shared derived character possess that character because the character arose once, in the common ancestor of the found at the node of the clade of interest. For example tool use is shared by chimps and homo, but it is not found in outgroups more removed from this sub-clade (gorillas or orangutans).
In the absence of fossil information (which is true for most species on the planet), how on earth do you make trees? You use the information from shared derived versus shared ancestral traits. The same principle applies to all kinds of information be it derived from molecules or morphology.
Willi Hennig is credited with coming up with a simple rule for reconstructing the evolutionary changes that have occured in a clade that has revolutionized the way comparative biology is carried. First let me contrast phylogenetic analysis before and after Hennig. In the dark ages, a professor would study a group, indeed they might even study all species in a group, and after such lengthy lifetimes work, they would draw a tree. The construction of the phylogenetic tree did not take place with any formal rules in mind. To learn how to draw such trees, students would enter into a lengthy apprenticeship of sorts and pick some smaller group of the large clade. In many cases the systematists would consider the concept of shared derived characters in the construction of their tree but no formal rule was used.
Willi Hennig formalized the use of shared derived characters by devising the principle of parsimony. The tree in which the fewest evolutionary steps are required to connect the different branches of a tree is considered the most parsimonious tree. In making such an assumption to connect branches of the tree, we assume that evolution is conservative and that evolutionary change does not occur all over the tree. Remember that any tree is our best guess as to the actual pattern of evolution underlying the phylogenetic relationships between species. A phylogenetic tree is a hypothesis of the pattern of evolution. Inferences is used in the construction of trees.
Let us examine a hypothetical example of how we would draw a the most parsimonious tree from the following traits and let us assume that ancestral is scored as 0 and advanced is scored as 1:
| trait 1 | trait 2 | trait 3 | trait 4 | |
| species A | 0 | 0 | 1 | 0 |
| species B | 1 | 1 | 1 | 1 |
| species C | 0 | 1 | 1 | 1 |
| outgroup | 0 | 0 | 0 | 0 |
The simplest way to construct the most parsimonious tree by "hand" is to identify pairs of species with the most derived set of characters as they have changed quite a bit, and to identify the species that have the most primitive set of characters. For example, species A only differs from the outgroup in a single trait, trait 3. In addition, the other two species also differ from the outgroup in trait 3, but they also differ in a number of other traits. Thus trait 3 distinguishes our clade of species A, B, and C from the outgroup and it also tells us that the branch from 1 to the outgroup should be closer to the ancestral node compared to the branches for species 2 and 3.
Now let us look at species 2 and 3. Species C has three derived traits, and species B has four derived traits. Again species C is closer to the node than species B, but it is farther from the node than species A. Voila, we have a tree based on the four traits and this tree has minimized the changes.
We can map the changes onto the tree with a "notch" and count that four changes are required. You can draw any other topology and you will require more changes than the four we see. Draw some other ancestor descendant relationship and test this out.
In practise, real data and real trees have conflicts between characters. One character suggests a different phylogeny than if we consider a different character. What if the distribution of traits among species was as follows:
| trait 1 | trait 2 | trait 3 | trait 4 | |
| species A | 0 | 0 | 1 | 0 |
| species B | 1 | 1 | 0 | 1 |
| species C | 0 | 1 | 1 | 1 |
| outgroup | 0 | 0 | 0 | 0 |
I have highlighted the single change in bold. Trait 3 and trait 1 provide a different set of trees. Now we have three primitive charcters for both species B and C and we cannot definitively place the node for these two species on the tree. There are two "most parsimonious trees", each of which requires 5 evolutionary steps. These two trees are, however, better than all other possible trees.
Such problems typically arise from either:
convergence in which the same character arises more than once on the tree (an abhorent thought to a strict adherent of the principle of parsimony)
or perhaps from evolutionary reversals where a derived character states reverts back to a more primitive state.
There are kinds of parsimony which allow for reversals in evolution so this is not much of a problem. However, multiple evolution of a characters on various branches of the tree is difficult for for the principle of parsimony. Consequently, the characters that people tend to choose are ones that minimize evolutionary reversals and the frequency of multiple evolution of characters which we refer to as homoplasy.
While, multiple evolution of character states or homoplasy, is bad from the point of view of constructing trees, it is a good thing from the point of view of the analysis of adaptations. If a trait evolves a number of times on a tree we have a much larger sample size to use in our tests of the conditions that drive the evolution of a trait. The more independent events that we observe the more data we have on the conditions that might favor the evolution of a behavioral trait.
This rule is simple to put into practise for a student of behavior. Do not use behavioral traits to make the tree. Do use behavioral traits as the focus of the study of adaptations.
Let's begin to put our ideas into operation with a not-so-hypothetical example taken from paleontology.
Many of you are familiar with a famous fossil find from Montana. Jack Horner has made dinosaur parental care nearly a household concept with his discovery of a dinosuar rookery that for all intents and purposes resembles the kind of rookery one might find in a seabird colony. Maiasaurus, which translates as "good mother", was found in very large numbers, and they placed there nest-like mounds of earth very close to one another.
They have also examined the fossil eggs and embryos using CAT scans and have noted a number of fossil dino "nestlings" for lack of a better word. From the bone structure of these nestlings, a functional morphology can argue that the nestlings were incapable of roaming the landscape. They have an altricial pattern of bone development. Altricial birds, like most songbirds participate in a phase of extended parental care. In altricial birds, the chicks are born relatively helpless, and the parents feed the young over an extended period. This life history pattern is contrasted with the development of most ducks in which the chicks hatch in a precocial developmental state, and they immediately leave the nest and forage on their own. The mother bird stays with chicks and guards her little flock of young, but the birds take care of their own feeding and are very capable of locomotion. The bone development of altricial and precocial birds is quite different. So from an argument based on functional morphology of fossil embryo and chick development, it is a pretty safe guess to infer that Maiasaurus nestlings were altricial and remained in the nest for a long period of time. At the very least, the mother tended to their needs.
Consider an argument based on pure comparative biology (phylogeny free):
In the vast majority of altricial birds, both parents participate in the rearing of young. This is not the case for precocial birds. Based on a straighforward argument of comparative biology, if altricial birds have female- and male-based care, then it is very likely that Maiasaurus males also had female- and male-based care.
Now lets consider a phylogenetic argument:
Dinosaurs do not have any extant living relatives -- or wait do they? It is generally agreed that birds arose from the midst of the dinosaur clade and could be considered the living relatives at the very least or an actual dinosaur if you take the arguments to the extreme.
The most ancestral dinosaur like form happened to be very crocodilian like, and it is generally agreed that crocodiles are more closely related to birds than they are to other living reptiles.
As an aside, this example illustrates an important point. While the systematics of vertebrates allies crocs with other reptiles, a phylogenetic analysis would place them more closely related to birds. The classification of crocs with other reptiles implies that the class reptilia is polyphyletic or a group that is comprised or members from different clades. It is not really a natural phylogenetic grouping of animals. Classification schemes are in a sense a hold-over from the bygone days of dark age phylogeny.
Now on to my main point. If we consider birds to be extant dinosaurs or at the very least the nearest living relatives of dinosaurs, then this suggests the possibility that some dinosaurs could have had female/male-based parental care and in particular, female/male-based is more likely in altricial birds. Next is there evidence for parental care in crocs. Yes females guard the nest, and when the little crocs hatch they begin vocalizing. The female uncovers the hatchlings and transports the young to the water. In some crocs even the males participate in this behavior and it appears that male and female crocs can protect their young for some time after they hatch. Because crocs are an outcrop in nearly every dino/bird phylogeny, this suggests that dinos undoubtedly had the capacity for male parental care. Male/female care in the outgroup and in an extant group strongly points to Maiasaurus as having both male and female care and I suggest that we revise the name to reflect this behavioral possibility.
Recall the definition of a Lek in which you find clusters of males, and females visit the lek primarily to mate with one male and then she leaves to care for her young.
A lek by definition has:
Fomwhere did leks evolve?
Hot spot hypothesis:
The hotspot idea makes some specific predictions regarding the likelihood of finding a lek evolving in birds. If females have large territories, a lek-based mating system is possible.
In contrast, if females have small territories males have to have large territories and no lek can be formed. Birds provide a wonderful group to study the evolution of lekking. One can measure the territory size of females and males. The prediction would be that leks occur in those species with large female home ranges, and small male home ranges.
Jacob Hogland plotted such relationships and found a perfect match of the occurance of leks for species in which males have small home range and females have large home ranges.
The next step would be to map some feature of the environment of each species onto the phylogeny and show correlated selective environment.
For example, in the original hotspot idea it was speculated that there might have been a resource that attracted females. Overtime the resource became unimportant relative to the resource provided by the ability of females to choose a male with good genes from among many males.
Sticklebacks, pipefish, and seahorses belong to the same family of fish and this group is noteworthy in the animal kingdom for the evolution of highly-advanced male care. We have already considered the role of ecology in shaping the mating system.This system is also illuminating with regards to the role of ecology in governing the evolution of mating systems.
The evolutionary relationships among these three groups is roughy as follows:
Sticklebacks -> Pipefish -> Seahorses
The morphological complexity of the male's brood pouch varies among the species comprising the clade of fish to which the seahorse belong. These differences have to do with the complexity, and quantity of nutrients that the male transfers to the developing embryos.
 |
 |
 |
 |
 |
male brood pouch |
copulation |
full brood pouch |
birth |
fry and father |
For example, in the outgroup of sticklebacks, the males have a certain amount of care, but all they really do is fan the eggs until they hatch -- there is no transfer of energy.
In pipefish males, the brood pouch is quite elaborate, but no real placential connections take place between the father and young.
Finally, the seahorses have the most complex brood pouch with an elaborate system for transfering nutrients.
The evolution of more derived (advanced) brood pouches in this groups maps quite well onto the phylogeny that has been described for the group.
We considered an experimental test of the predators propensity to learn whether prey were aposematically colored within a very short time frame, and whether being solitary or gregarious gave the evolution of Aposematic coloration a selective boost. Recall Ronald Fisher's original arguments:
Ronald Fisher observed that many aposematic forms tend to also be quite gregarious and congregate in the same locale. Fisher speculated that kin selection may favor such aggregations. An individual may die during the lesson required to teach a naive predator that the color also results in a bad experience. However, because the predator leaves the remaining kin alone, the inclusive fitness of the dead aposematling is positive because the cost of individual death is balanced by the surviving kin that are left alone. Gregariousness can easily result from kin groups (e.g., a localized clutch), and such kin groups greatly enhance the probability that aposematic coloration will spread even though brightly colored individuals attract attentions of naive predators.
Sillen-Tullberg studied the evolution of aposematic coloration by mapping both the morphological trait (bright color as a proxy for unpalitability) and the behavioral trait (gregariousness) onto trees. She was more interested in whether the origin of gregariousness was contigent upon unpalatability and aposematic coloration evolving first. The evolution scenario for her arguments go as follows:
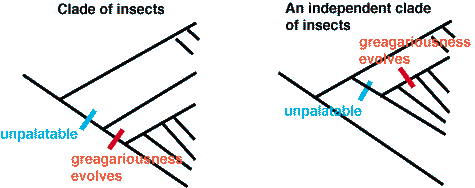
Sillen-Tullberg tested these ideas out on several clades of caterpillars. Unfortunately unpalatability is very difficult to score (you have to make a lot of birds barf) -- so she used aposematic coloration as a conservative index of unpalatability. She found that unpalatability did preceed gregariousness every time. Gregariousness is a behavior of the female -- she decides to lay one or many eggs on a plant.
Recall the conditions for the evolution of hostplant toxicity and the sympatric speciation of their herbivores. This example serves us with a classic case of coevolution. Coevolution in insects and plants relates to the "endless evolutionary arms race" which leads to (Erlich and Raven, 1964 cited in Farrell and Mitter 1994):
I have cartooned the idealized clades for hostplant and herbivore below.
Note that there is a perfect correspondence between clades in the ideal
case. Nature presents us with some near perfect examples.
I will discuss Farrel's data on the coevolution between beetles and their milkweed hosts. The beetles have a tight association with their hosts which is required for true coevolution and eggs in that larvae and adults require the milkweed.
We can contrast the beetle associations with butterflies, that only feed on nectar as adults. The offspring of butterflies are raised on milkweed, but because adults are not necessarily dependent on the milkweed for food, the coevolution is not seen in the butterfly/milkweed phylogenies.
Farrell, B. D. and C. Mitter., 1994. Adaptive radiation in insects and plants: time and opportunity. Amer. Zool. 34:57-69.
Few studies of organisms allow one to test all the ingredients necessary to discriminate between the competing hypotheses of runaway sexual selection and good genes models. One of the key pieces of evidence missing in all of the previous studies is the phylogenetic history of sexual selection. How do we know that a male trait under sexual selection in the present-day as an indicator of male quality evolved specifically for the purpose of an advertisement of quality? If a trait evolved for a specific function, then we can refer to the trait as an adaptation that solves a problem of sexual or natural selection. The origin of a trait makes individuals in the species better adapted to their environmental conditions. When we consider the evolutionary origin of a trait, we are delving into ultimate issues that define why a trait evolved. How females choose males and what sensory systems are used, is an issue of proximate mechanism.
We might search for the answer of a trait's origin in the fossil record to get at the evolutionary history of a group, but female choices do not fossilize and many male ornaments are far to delicate to leave a trace in the rocks. We need flesh, not just bones, to get at the dynamics of sexual selection and mate choice. In recent years, behaviorists have turned to a new branch of the comparative method called phylogenetic reconstruction (Brooks and McLennan 1991). While I cover the subject of phylogeny and history more thoroughly in an upcoming chapter (Chapter 17), I will go over some of the basics here. A phylogeny is a family tree of relationships that describes the degree to which modern day species are related. The phylogeny is our best guess as to which modern species are most closely related to one another, and which might be most distantly related. On a phylogeny, species that are separated by long branches are less closely related compared to species that are separated by short branches.
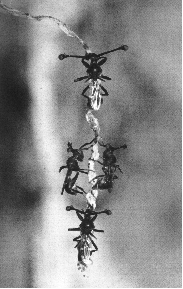 Figure 10.25. Courtship in stalk-eyed flies involves displays by males
in a lek. Females tend to choose males with the largest stalks. Leks tend
to form on the root hairs of plants. (From (Wilkinson and Reillo 1994).
Figure 10.25. Courtship in stalk-eyed flies involves displays by males
in a lek. Females tend to choose males with the largest stalks. Leks tend
to form on the root hairs of plants. (From (Wilkinson and Reillo 1994).
For example, there are three species of flies that have eyes located on stalks that are quite closely related to each other in a phylogenetic sense (Wilkinson et al. 1998). One of the three, Cyrtodipsis quinqueguttata is more distantly related to the other two, C. whitei and C. dalmanni. In both C. whitei and C. dalmanni, males possess eyestalks that are far longer than the female's eyestalks. In third species, C. quinqueguttata, the eyestalks of males and females are the same length. A difference between the sexes or the degree of sexual dimorphism is our first clue that eyestalk length is a sexually selected trait in the males. Wilkinson and colleagues (1998) tested the proposition that males of the two sexually dimorphic species use the eyestalks to attract females. They conducted classic female choice experiments where the female chooses between a short and long males, and manipulative experiments where eyestalk length was varied by the experimenter. Both sexually dimorphic species showed dramatic female choice for long eyestalks, but the monomorphic species showed no female choice for variation in eyestalk length (Wilkinson et al 1998). Eyestalks are sexy in two species of stalk-eyed flies but not the third.
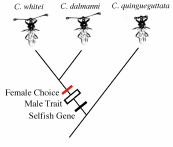 Figure 10.26. Phylogenetic relationships among species of Stalk-eyed
flies in the genus Cyrtodipsis. Cyrtodipsis whitei and C. dalmanni
share more DNA base pairs in common and probably derived more recently
than C. quingueguttata. Both of the two closely related species have
the sexually dimorphic male trait, exhibit female choice for long eye stalks,
and also possess a selfish gene that causes sex ratio changes in progeny.
The parasitic selfish gene is the change in enviroment leading to the origin
of sexual seleciton. The monomorphic species exhibits none of the traits.
A parsimonious reconstruction of trait evolution places all three
changes at the same poin. C. whitei and C. dalmanni inherit
the traits from a common ancestor (see text) (redrawn from Wilkinson et
al 1998).
Figure 10.26. Phylogenetic relationships among species of Stalk-eyed
flies in the genus Cyrtodipsis. Cyrtodipsis whitei and C. dalmanni
share more DNA base pairs in common and probably derived more recently
than C. quingueguttata. Both of the two closely related species have
the sexually dimorphic male trait, exhibit female choice for long eye stalks,
and also possess a selfish gene that causes sex ratio changes in progeny.
The parasitic selfish gene is the change in enviroment leading to the origin
of sexual seleciton. The monomorphic species exhibits none of the traits.
A parsimonious reconstruction of trait evolution places all three
changes at the same poin. C. whitei and C. dalmanni inherit
the traits from a common ancestor (see text) (redrawn from Wilkinson et
al 1998).
The phylogenetic question relates to the origin of the male trait, and the origin of the female choice. Are eyestalks a sexually selected trait that has evolved to solve a new problem faced by Cyrtodipsis whitei and C. dalmanni. If so, these two species should have arisen quite recently relative to the monomorphic species C. quinqueguttata. We can think of eyestalks and female choice as derived traits relative to the more ancestral monomorphic condition seen in C. quinqueguttata. The phylogeny for the three species indicates that the branch length for C. quinqueguttata reaches deeper into the past compared to the more recent origin of the sexually dimorphic species, C. whitei and C. dalmanni. We can map the evolutionary changes in eyestalk length and female choice onto the phylogenetic tree. The simplest hypothesis would be that the monomorphic condition is ancestral (quite a logical one, I might add), and that the sexually dimorphic condition arose once, when the hypothetical ancestor of the two sexually dimorphic species split off from the ancestral monomorphic species. This places the origin of the traits before the two species split off from one another, but after the two split off from the monomorphic species (Fig. 10.26).
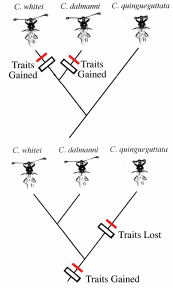 Figure 10.27. Alternative reconstructions of trait evolution for the
origin of long eye stalks (open box) and female choice (dash). While each
of the two phylogenies is plausible, the reconstructions of trait origin
(or loss) would require twice as many evolutionary steps as that found in
Figure 10.26.
Figure 10.27. Alternative reconstructions of trait evolution for the
origin of long eye stalks (open box) and female choice (dash). While each
of the two phylogenies is plausible, the reconstructions of trait origin
(or loss) would require twice as many evolutionary steps as that found in
Figure 10.26.
We have just reconstructed a plausible evolutionary history for female choice and the male trait using information from the distribution of traits among modern-day species, and the phylogenetic relationships among modern-day species. In our hypothesis of trait origin we assumed that the change between the ancestral monomorphic species and the two more derived dimorphic species only occurred once; this would be the simplest hypothesis. Using this assumption is also referred to as the principle of parsimony. Parsimony assumes that evolutionary change is slow enough that more complex hypotheses of trait origin are far less likely than the simplest hypothesis. For example, the two dimorphic species could have acquired the traits in two separate origins. Alternatively, all three species could have acquired the trait through a common ancestor, but the monomorphic species subsequently lost the dimorphic condition. The last two more complex hypotheses would require twice as many total evolutionary steps than the parsimonious hypothesis. In the absence of any other information, the parsimonious hypothesis is taken to be a 'null hypothesis' for the distribution of traits in the phylogeny.
With a working hypothesis in place of when male trait and female choice arose in the phylogeny of stalk-eyed flies, we move on to the next question: why did eye stalk length evolve in the two sexually dimorphic species? To address this question, we need to reconstruct the selective environment that might have led to the stalk-eyed trait being useful in males as an indicator trait for females. Again, there is no fossil record for reconstructing the conditions that led to sexual selection for long eye stalks. Fortunately, there is genetic evidence of a major change that would alter the mating environment of the flies (Fig. 10.24). Its time to synthesize some previously discussed issues of genic selection (Chapter 4) and sex ratio evolution (Chapter 9). The two sexually dimorphic species both possess a selfish gene on the sex-determining X-chromosome that for lack of a better word, kills off the Y during meiosis and replaces the missing chromosome with a copy of the X that carries the selfish gene. Like all selfish genes, all it does is over-reproduce itself during meiosis such that males who are infected with the element tend to produce more X-sperm that carry the element than Y-sperm (see Chapter 4, t-alleles in mice). This causes a male carrying the selfish gene to produce sperm that carry X-chromosomes. A female that mates with an 'infected' male would produce mainly daughters.
The selective consequences of this are straightforward, if we recall that the theory of Fisherian sex ratio favors a 50:50 sex ratio. Species "infected" with the selfish gene produce female biased sex ratios in both nature and laboratory cultures. Females that produce a biased sex-ratio of female offspring are at a striking disadvantage. Fisherian sex ratio theory indicates that a genotype which produces a 50:50 sex is evolutionarily stable (see Chapter 8), and the presence of sex ratio bias in stalk-eyed flies means that mothers with female-biased sex ratios are producing lots of female progeny that will have trouble finding mates. A female--biased population is susceptible to invasion by a female that can produce a male-bias. Accordingly, any gene that restores the biased sex ratio in males back to a 50:50 ratio of X:Y sperm would cause 'discriminiating' females to produce sons and thereby give their offspring a mating advantage relative to females that mate indiscriminately. Sons would have lots of females to mate with. Such a gene has evolved on the Y-chromosome and this Ym gene negates the effects of the selfish gene located on the X-chromosome, Xd, that distorts sex-ratio. Interestingly, the genes controlling long eye stalks in males is closely linked to the Ym . Females use the stalk-eyed trait in males as an indicator of their superior genetic background in that males with long eye stalks are more likely to bear the Ym gene, a gene that restores the sex ratio of their progeny back to a 50:50 sex ratio.
The natural history and genetics of the stalk-eyed flies is the first demonstration of a sexually-selected male trait evolving to serve as an indicator of a male's genetic quality. This story has all the elements that are required to support the theory of good genes. The correlation between female choice and the male trait is present in the sexually dimorphic species. When laboratory stocks of the fly are selected for longer or shorter stalks, female choice evolves in a correlated fashion (Wilkinson and Reillo, 1994). Moreover, the stalk-eyed male trait is genetically linked to a gene that rescues individuals from the effects of a selfish genetic element. Finally, eye stalks orginate as an adaption to indicated male quality.
Wilkinson et al (1998) have suggested that many male Y-linked traits such as guppy spots may be commonly used by females to indicate the presence of selfish genetic elements. Selfish genetic elements may be quite common in nature since distortions in sex ratio are found in guppies, mice (see t-allele example, (Lewontin 1962), and Drosophila (Atlan et al. 1997). Female choice for males may be driven by the ever present force of selfish genes, of which one large class of "sex-ratio-drivers" serves to distort the primary 50:50 sex ratio that is evolutionary stable in the long run. For example, male mice that are heterozygous for the t-allele (+|t, discussed in Chapter 2) likewise produce a distorted ratio of sperm bearing t-allele. Any female mouse with a heterozygous genotype (+|t) will produce sterile sons if they mate with a heterozygous male (t|t causes sterility in 1/4 of her sons). Accordingly, females with a +|t have evolved mate discrimination that allows them to avoid mating with heterozygous males in favor of wild type males (+|+) that are uninfected with the selfish t-allele. The case of infections by selfish genes and female discrimination of males that carry the bad gene (e.g., mice) or carry a good gene (e.g., stalk-eyed flies) is certainly not the only situations in which indicator genes might prove useful. However bizarre, this example serves to highlight the power of sexually selected processes to couple male traits with female choice in the face of a dramatic infection of the genome by a 'parasitic gene'.
Theories of sensory bias postulate that the evolution of a sexually selected male character arises in a group in which females have a pre-existing phylogenetic bias for certain kinds of signals, and those signals are the ones that males evolve. The basic phylogenetic distribution for the female preference and male trait is as follows:
The principle of parsimony is crucial because the argument above assumes that evolution occurred in the smallest number of changes.
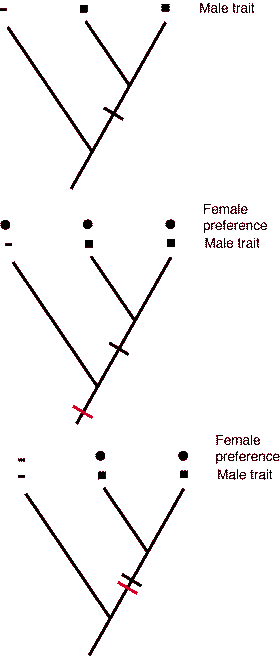 |
Hypothetical relationship between a male and the phylogeny. Notice that the male trait does not occur in the outgroup. The mapping of a single evolution for the male trait assumes that it only evolved once -- it could have evolved twice, once in each lineage. |
| Hypothetical relationship between a male trait and female preference that would suggest that preference evolved prior to the male trait. (Note, a non-parsimonious reconstruction could have preference evolve three times! | |
| Hypothetical relationship between a male trait and female prefernce in which the hypothesis of correlated evolution of the trait and preference cannot necessarily be ascribed to a pre-existing bias. |
Recently, behaviorists have applied phylogenetic techniques to test the order in which female choice originates in relation to the origin of a male trait. The case of the stalk-eyed flies provides support for the idea that the male trait and female choice evolved nearly simultaneously in response to a change in mating environment; a biased sex ratio. A recent theory (Endler 1992; Ryan and Rand 1993; Ryan 1997) relates to sensory biases in the nervous or sensory system of females that pre-disposes them to pick males for some traits over other males that lack the trait. Females choose males not because they perceive them as sexy per se, but because they are "attracted to them". Such biases are present in the ancestral species and they remain latent in a population until a male evolves a mutation. Because a mutation in ornament "exploits" pre-existing sensory bias found in females, the theory of sensory bias is also referred to as sensory exploitation. Certain stimuli (e.g., colors, shapes, movement) may be useful in certain contexts (e.g., feeding and foraging) and the nervous system of females (and males) is honed by natural selection to be efficient at picking out food items from a world that is overly rich in extraneous stimuli. In a sense, these parts of the nervous and sensory system may be co-opted by sexual selection and a mutant male that displays a trait that triggers a heightened response in females may have an advantage. A male's signal may become fine-tuned such that it maximally stimulates the female sensory system.
Theories of sensory bias postulate that sexually selected male traits evolve in a species where females have a pre-existing phylogenetic bias for certain kinds of signals. Evidence favoring the idea of a sensory bias would place the origin of female choice as an ancestral condition (e.g., occurring earlier) relative to the origin of the male trait. In this chicken and egg argument, the evolution of female choice precedes the evolution of the male trait.
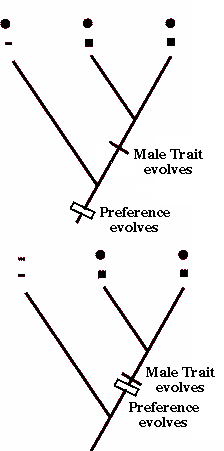 Figure 10.28. Hypothetical relationships between a male trait and
female preference with regard to their distribution in the phylogeny. The
phylogeny describes ancestor descendant relationships between the three
extant (modern-day) species. The distribution of the male trait in extant
species is denoted with a square. The presence of the female preference
is denoted by a circle. Absence of the traits is denoted by a minus. Notice
that the male trait does not occur in the more deeply branching outgroup
in either clade. The outgroup species presumably reflects the ancestral
condition of the trait. a) Hypothetical relationship between a male trait
and female preference that would suggest that preference evolved prior to
the male trait. b) Hypothetical relationship between a male trait and female
preference in which the hypothesis of correlated evolution for the male
trait and female preference cannot necessarily be ascribed to a pre-existing
bias (from (Sinervo and Basolo 1996).
Figure 10.28. Hypothetical relationships between a male trait and
female preference with regard to their distribution in the phylogeny. The
phylogeny describes ancestor descendant relationships between the three
extant (modern-day) species. The distribution of the male trait in extant
species is denoted with a square. The presence of the female preference
is denoted by a circle. Absence of the traits is denoted by a minus. Notice
that the male trait does not occur in the more deeply branching outgroup
in either clade. The outgroup species presumably reflects the ancestral
condition of the trait. a) Hypothetical relationship between a male trait
and female preference that would suggest that preference evolved prior to
the male trait. b) Hypothetical relationship between a male trait and female
preference in which the hypothesis of correlated evolution for the male
trait and female preference cannot necessarily be ascribed to a pre-existing
bias (from (Sinervo and Basolo 1996).
Alexandra Basolo looked at a large genus of fish, Xiphoporus, which have evolved elongated swords on their tail fins (Basolo 1990; Basolo 1995). The sword-tail is used as a sexual ornament. In species where males possess a sword, females prefer males with long swords (no surprise). A phylogeny of Xiphophorus indicates that many species have derived swords. One member of the genus, X. maculatus, has the "ancestral" condition and lacks a sword. Females in this species are quite content to mate with their swordless males; at least until Basolo tempted them with sworded males. Basolo gave females from this ancestral species a choice between males of their own species which lack a sword, or males of their own species with a surgically attached a sword. To control for the effect of surgery on the swimming ability of the male, which might interfere with his courtship, the first group of control males lacked a visible sword, but they did receive a clear sword tied onto the base of the tail fin. The sworded males received an opaque sword tied on to the base of the tail fin. She placed the two males in a pairwise choice trial and females overwhelming choose males that had a sword tied on!
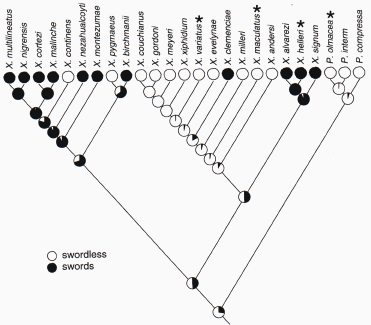 Figure 10.29. Distribution of sworded males in the genus Xiphophorus
and Priapella. Because the females in species whose males normally
lack swords (denoted by *) prefer males with swords, Basolo inferred that
the preference for swords arose as a pre-existing bias. The "pie-diagrams"
below the tips of the tree reflect the best guess condition (probability
of sword is proportional to area of black) for the male trait in hypothetical
common ancestors that are located at the nodes joining the tips (from (Schluter
et al. 1997)).
Figure 10.29. Distribution of sworded males in the genus Xiphophorus
and Priapella. Because the females in species whose males normally
lack swords (denoted by *) prefer males with swords, Basolo inferred that
the preference for swords arose as a pre-existing bias. The "pie-diagrams"
below the tips of the tree reflect the best guess condition (probability
of sword is proportional to area of black) for the male trait in hypothetical
common ancestors that are located at the nodes joining the tips (from (Schluter
et al. 1997)).
Further work on a more remote ancestral species Priapella olmacea. indicated that female preference evolved quite early in the history of this group of fish.Once again, females prefer their own males with swords tied on, even though males do not naturally possess swords. Basolo interpreted the female preference for swords in two swordless species in terms as an "ancestral" or pre-existing bias for sworded males in these fish. The idea also explains the widespread distribution of swords in other species of the genus.
Species in the hypothetical ancestor had a pre-existing bias for the sword, and when this trait showed up in some descendant, the sword spread through the population like wildfire. The exact reason for a pre-existing bias in the fish is unclear. Basolo has suggested that the sword may resemble the males gonopodium, which is a penis used to fertilize the eggs in the female's brood pouch. This group of fish has internal fertilization and brood eggs in a female brood pouch. Females will only mate with a male that displays readiness in the form of an extended gonopodium. Basolo hypothesizes that the sword provides a supernormal stimulus that heightens the female's readiness for copulations. Support for the sensory stimulation hypothesis, is provided by strength of female preference in species which possess swords. In these species, the females prefer supernormal stimuli over normal length swords. More work needs to done on the exact sensory mechanisms that predispose female swordtails to choose males with elongate tails.
The tungara frogs provide evidence that males co-opt specific mechanisms for sound reception in the female ear. Ryan and his colleagues have investigated a similar case of phylogenetic sensory bias in the tungara frog of Central and South America (Ryan and Rand 1993). This frog collects at ponds and uses a call to attract females. In a simple experiment, the composition of songs can be digitally altered on the computer and then played through speakers. Females readily respond to the songs being played from a speaker. Positive female choice was scored by movement of the female towards one speaker or the other. Males in two of the species, Physalaemus petersi and P. pustulosus, possess a more complex call consisting of two parts: an initial whine, followed by a chuck. Males in the other two species, P. coloradorum and P. pustulatus, do not possess a chuck at the end of the song, but only call with the whine component.
To generate the synthetic chucks in species with chuckless males, Ryan and his colleagues took the species typical whine and digitally mastered a chuck at the end of the song. Choice experiments indicate that females in all species prefer males that have a chuck added at the end of the song, regardless of whether or not the males of their species possess a chuck. Thus, available phylogenetic evidence suggests that the ancestral species consisted of females that had a preference for the chuck. The innovative chuck arose in one branch of the phylogeny, presumably by a mutation, and the ensuing chuck spread through the population because females already had a pre-existing preference for the chuck.
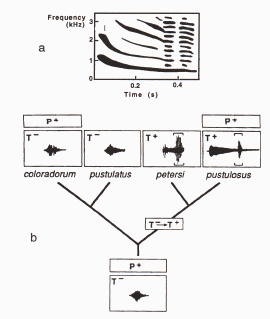 Figure 10.30. a) A sonogram of the mating call of male tungara frogs,
Physalaemus spp., containing a whine (long horizontal lines) and
two chucks (stacked lines at the end). b) The small panel for each species
gives the amount of energy in the call over time. The high energy chuck
is restricted to the end denoted by brackets. The distribution of female
preference and male traits in the phylogeny of tungara frogs: P+ preference
present, T+ male chuck present, and T- male chuck absent. Even though the
males of the two species considered to be more ancestral, P. coloradorum
and P. pustulatus, do not use a chuck in their calls, females
of these species appear to have an ancestral bias or pre-existing predisposition
for males with the chuck. Thus, the existence of a chuck in the derived
calls of P. petersi and P. pustulosus are thought to have
originated because the hypothetical common ancestor at the root of the tree
had females with the pre-existing preference. (from (Kirkpatrick and Ryan
1991).
Figure 10.30. a) A sonogram of the mating call of male tungara frogs,
Physalaemus spp., containing a whine (long horizontal lines) and
two chucks (stacked lines at the end). b) The small panel for each species
gives the amount of energy in the call over time. The high energy chuck
is restricted to the end denoted by brackets. The distribution of female
preference and male traits in the phylogeny of tungara frogs: P+ preference
present, T+ male chuck present, and T- male chuck absent. Even though the
males of the two species considered to be more ancestral, P. coloradorum
and P. pustulatus, do not use a chuck in their calls, females
of these species appear to have an ancestral bias or pre-existing predisposition
for males with the chuck. Thus, the existence of a chuck in the derived
calls of P. petersi and P. pustulosus are thought to have
originated because the hypothetical common ancestor at the root of the tree
had females with the pre-existing preference. (from (Kirkpatrick and Ryan
1991).
The sensory bias or pre-existing bias found in tungara frogs has a mechanistic
basis in the vocal apparatus of the amphibian ear. The mechanics of the
auditory apparatus of the amphibian ear also explains the nearly universal
preference that females frogs have for large bodied males. As might be expected,
larger males can produce lower-pitched calls than smaller males. These kinds
of calls are much more effective at stimulating the female's ear. Specifically,
the sound waves enter the female's ear and stimulate a cluster of receptors
referred to as the basal papilla, which are sensitive to the range of sounds
in lower frequencies. The females also possess an organ called the amphibian
papilla which is responsible for fine scale discrimination of sound frequencies.
The amphibian papilla is present in the ears of all species of tungara frogs,
and the amphibian papilla is maximally stimulated by the frequencies produced
by the chuck at the end of the call. Why this derived auditory structure
is present in tungara frogs is not known, however, the evolution of the
structure would have predisposed this group to the evolution of a male type
that could exploit the pre-existing sensitivities of the female ear.